Can the MarginProbe Device Improve Breast Cancer Surgery?
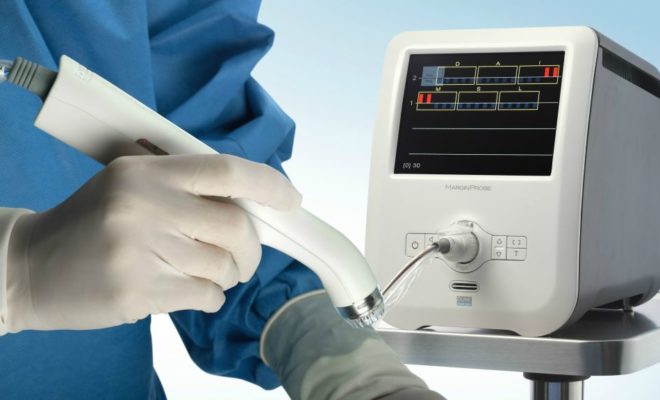
The MarginProbe device has been used in breast cancer surgery for the past five years, and it has proven to be a valuable tool for surgeons. The device can detect microscopic residual cancer on the surgical margin using radiofrequency spectroscopy. As a result, this device allows surgeons to take additional tissue from the patient after surgery, reducing unnecessary surgeries. But how can surgeons determine whether this device will improve their care?
Fortunately, it has been used in over 100 hospitals in the US and 12 in Israel. The new device has been clinically tested on over 10,000 patients, and it is being evaluated by the Israeli Health Ministry to become standard care in the country. This would allow hospitals to receive reimbursement for using the device. Unfortunately, the lack of reimbursement has hindered the growth of the technology in Israel. However, this could change as the FDA evaluates the device in more trials.
One of the significant benefits of the MarginProbe is its ability to identify cancer in real-time during surgery. In addition, the technology is integrated into the operating room, and the system has been found to increase the probability of a clean margin. According to a recent study, more than 99% of patients have clean margins following a lumpectomy. And, despite the high cost, it is a highly effective tool.
The MarginProbe device has been approved by the FDA and is currently used by over 100 hospitals in the US and 12 hospitals in Israel. In addition, it has been commercially used on more than 10,000 patients. In Israel, Dune Medical is in talks with the Health Ministry to have the probe recognized as standard care so that hospitals can be reimbursed for using it. This is a significant issue for the device in Israel. It is unclear if it will be adopted there, but the company remains hopeful.
The MarginProbe has been used in multiple studies, and it has proven to be an effective tool for detecting malignant tissue. In a recent study, the device reduced re-excision rates by 57%. In addition, its usage increased tissue volume during the primary and secondary lumpectomy. In addition, users removed less tissue than control patients. Unfortunately, the MarginProbe device is not an exception to these limitations. It has been a breakthrough for breast cancer research, but it also has a lot of drawbacks.
The MarginProbe has been approved by the National Institute of Health for use in cancer surgeries. Its FDA-approved technology makes it possible for surgeons to identify cancer cells with high precision. In a previous study, it was only possible for a small group of women to benefit from the device, but it may also be helpful in other cases. It is not recommended for use in all patients, but it can significantly reduce the chances of re-excision in a large number of issues.
The MarginProbe device has been evaluated in clinical studies. Three major randomized studies were conducted to compare their effectiveness in this study. In these trials, the devices significantly improved resection and re-excision rates. In addition to these results, the marginProbe has also been used in several other procedures, including breast cancer surgery. It is available in various sizes. If you consider the device for your breast cancer surgery, make sure it meets the criteria listed above.
A single-use probe is a valuable tool for detecting cancer in the margin of a breast tumor. Its sensor-equipped instrument is similar to an ultrasound instrument. The surgeon uses the margin probe to scan the tissue around the cancer. Its reflected signals can tell whether or not there are any cancer cells. If the margin probe does detect cancer, the procedure is completed more efficiently. So, the surgery is more affordable.
The margin probe device is a single-use probe attached to a single-use search. The investigation looks like a large pen. The surgeon inserts the instrument into the breast after the tumor has been removed. The sensors on the surgical margin send signals to the tissue, which can indicate whether there are any cancerous cells. The FDA has approved the surgical margin probe, but hospitals have yet to purchase it.












